Recent Literature: Efficacy, Safety & Use of Gelmix & Purathick Thickeners
This post is intended as a literature guide for healthcare professionals. Please always consult your healthcare professional before thickening liquids to ensure it is safe and appropriate for your or your child’s individual needs.
Efficacy & Implementation
Duncan, D. R., Larson, K., & Rosen, R. L. (2019). Clinical Aspects of Thickeners for Pediatric Gastroesophageal Reflux and Oropharyngeal Dysphagia. Current gastroenterology reports, 21(7), 30. https://link.springer.com/article/10.1007/s11894-019-0697-2
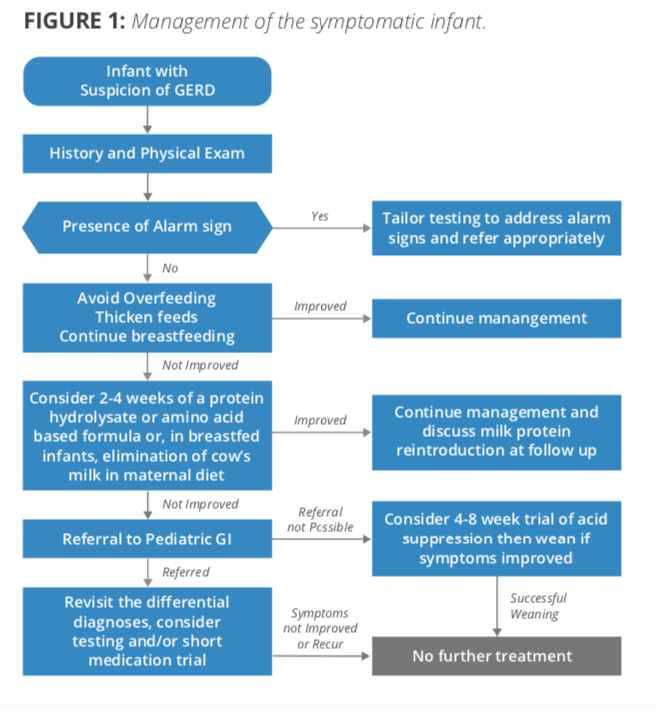
This article reviews “current knowledge and recent findings regarding clinical aspects of thickeners for pediatric gastroesophageal reflux and oropharyngeal dysphagia.”(p.29) Finds that, “thickeners are effective in decreasing regurgitation and improving swallowing mechanics and can often be used empirically for the treatment of infants and young children.”(p.29)
Rosen, Rachel, et al. “Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition.” Journal of pediatric gastroenterology and nutrition 66.3 (2018): 516-554. https://www.naspghan.org/files/Pediatric_Gastroesophageal_Reflux_Clinical.33.pdf
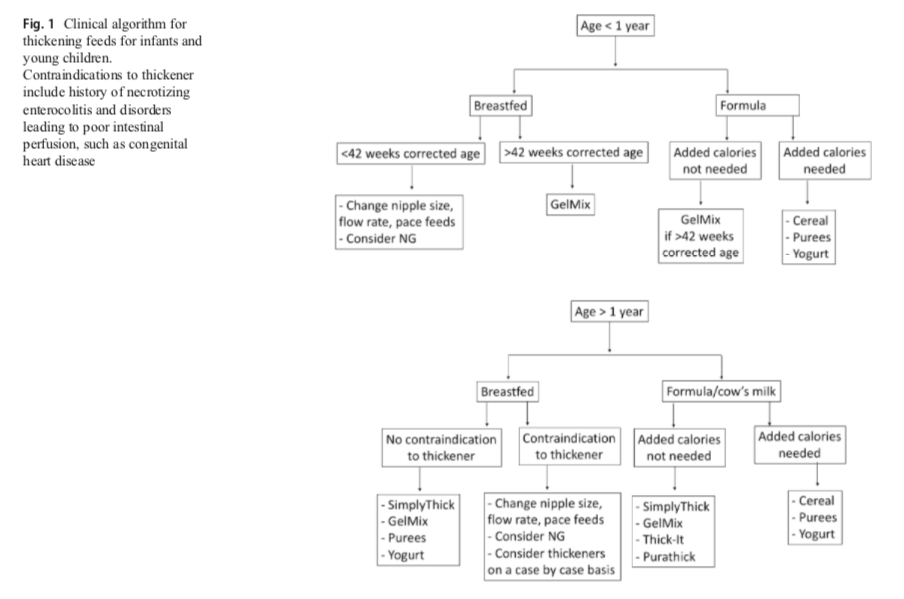
“In the vast majority of infants presenting with physiological GER, breastfeeding should be further encouraged. However, for babies with significant reflux such that thickening is being considered, breastmilk can be thickened with xanthum gum or carob bean based thickeners but not with cereal, the latter of which is digested by the amylases in breast milk. While these commercial thickeners are available for use in breast milk, some cautions exist. Carob bean thickeners are approved for use in infants after 42 weeks gestation. Xanthum gum thickeners are approved for infants greater than 1 year old because of concerns of necrotizing enterocolitis” (p. 534)
Summary of the 2018 NASPGHAN-ESPGHAN Pediatric Gastroesophageal Reflux Clinical Practice Guideline (for primary care physicians/pediatricians)
https://naspghan.org/files/documents/pdfs/position-papers/PedGERD%20Summary_Short_5-24-19%20-%20FINAL.pdf
Koo, Jenny K., et al. “Through Thick and Thin: The In Vitro Effects of Thickeners On Infant Feed Viscosity.” Journal of Pediatric Gastroenterology and Nutrition (2019).
Study using various thickeners with donor human milk and formula. Key findings: Gum based thickeners (xanthan and carob bean) effectively thickened both human donor milk and formula, however xanthan gum based thickeners achieved notably higher viscosities than intended based on manufacturer recommendations. Human donor milk and formula thickened with Gelmix achieved target viscosities following manufacturer recipes. Adding acid to xanthan-gum thickened donor human milk resulted in phase separation and formation of solid precipitant. Theorizes this may contribute to development of NEC and other gastrointestinal issues. Gelmix notably did not exhibit this phenomenon in the presence of acid. Starch based thickeners were unable to thicken donor human milk, even in acidic environment. The use of Gelmix, carob bean gum based thickener, may be preferable to reduce symptoms of GERD.
Fogleman, C., and L. Kobrin. “Feed Thickener for Newborn Infants with Gastroesophageal Reflux.” American family physician 98.5 (2018): 275-276. https://www.aafp.org/afp/2018/0901/p275.pdf
Evidence based review showing that “feed thickeners decrease the number of reflux episodes in full-term formula-fed infants” and “full-term formula-fed infants with GER who are given thickeners are more than twice as likely to be asymptomatic compared with infants not receiving thickeners at one to eight weeks of follow-up (p. 275).
Theophilou, I. C., C. M. Neophytou, and A. Kakas. “Carob and its Components in the Management of Gastrointestinal Disorders.” Journal of Hepatology & Gastroenterology. Volume 1, Issue 1 (2017): 1-005. http://www.scientificoajournals.org/pdf/jhge.1005.pdf
“The advantage of carob-based thickening agents, compared to other agents, is that the former are not broken down by salivary amylases and are able to maintain their thickening properties after reaching the stomach. Furthermore, the energy distribution of the infant’s food formula is not altered by the carob-based thickening agents because these agents do not alter the nutritional value of the food” (p. 002).
Gosa, Memorie M, The University of Alabama & Druid City Hospital. “Effect of Commercially Available Thickening Agents on Ready to Feed Infant Formulas.” Clinical poster prepared for ESSD (2017). https://www.postersessiononline.eu/173580348_eu/congresos/ESSD2017/aula/-S13-3.5_1_ESSD2017.pdf
Using Line Spread Test method of testing, this study finds Gelmix mixed with ready to feed formulas to be thinner than expected for therapeutic use for dysphagia. **NOTE: Due to other similar independent findings, Parapharma Tech updated the Gelmix mixing instructions & dosage guidelines in 2018 to standardize and be compliant with IDDSI (International Dysphagia Diet Standardisation) flow test and labeling.**
Koo, Jenny K., Lars Bode, and Jae H. Kim. “Current methods of thickening feeds for preterm infants with gastroesophageal reflux disease is highly variable.” The FASEB Journal 30.1_supplement (2016): 151-5. https://www.fasebj.org/doi/abs/10.1096/fasebj.30.1_supplement.151.5
Gelmix maintained thickening potential in human donor milk compared to rice cereals, starch based thickeners and xanthan gum based thickeners.
McSweeney, Maireade E., et al. “Oral feeding reduces hospitalizations compared with gastrostomy feeding in infants and children who aspirate.” The Journal of pediatrics 170 (2016): 79-84. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4769944/pdf/nihms-738149.pdf
“Patients who underwent gastrostomy tube placement for the treatment of aspiration had two times as many admissions as compared with aspirating patients fed orally. We recommend a trial of oral feeding in all children cleared to take nectar or honey thickened liquids prior to gastrostomy tube placement.” (p.1)
Eney, Anna, UW Nutritional Sciences Program, MS-Nutrition Student & Dietetic Intern. “Changes in Pediatric Hospital Wide Thickening Protocol in Response to Events and Evidence.” Clinical poster prepared for: MNT School of Public Health at University of Washington. (2015). http://depts.washington.edu/nutr/wordpress/wp-content/uploads/2015/08/Eney_MNT-Poster_FINAL-DRAFT.pdf
Study shows changes in Seattle Children’s Hospital thickening protocols from 2011 to 2015. In 2011, Gel-based thickeners (i.e. Simply Thick) were used with breastfed, non-premature infants (>37 weeks). In 2015, no thickeners were recommended for infants on breast milk or formula under a corrected gestational age of 42 weeks. For breastfed infants with corrected gestational age >42 weeks and weighing at least 6 pounds, Gelmix is listed as an option. For formula fed infants with corrected gestational age >42 weeks and weighing at least 6 pounds, cereals, Thick-it and Gelmix are listed as options.
Cichero, Julie AY. “Thickening agents used for dysphagia management: effect on bioavailability of water, medication and feelings of satiety.” Nutrition Journal 12.1 (2013): 54. https://nutritionj.biomedcentral.com/track/pdf/10.1186/1475-2891-12-54
Study shows that thickeners do not affect water bioavailability, however bioavailability of medications is impaired with viscous substances.
Safety of Ingredients: Gelmix
Gelmix is contraindicated for low birth weight and premature infants until they weigh a minimum weight of six pounds and have reached a corrected age of 2 weeks (42 weeks post menstrual age).
Gelmix is made from three simple hypoallergenic ingredients, organic tapioca maltodextrin, organic carob bean gum and calcium carbonate, all with a long history of safe use with infants. Tapioca maltodextrin is an easily digestible polysaccharide, used as a food additive, derived from the cassava root. Tapioca maltodextrin is often found in hypoallergenic infant formulas, and maltodextrin is the most common source for carbohydrates in enteral formulas. Maltodextrin is also a common ingredient in commercial thickeners, though usually derived from corn or wheat. Gelmix only uses maltodextrin derived from tapioca. Carob bean gum, also known as locust bean gum, is a natural gum extracted from the seed of the locust bean tree and is the second ingredient in Gelmix. Carob bean gum has been safely utilized for the preparation of breast milk and infant formula in the dietary management of infant regurgitation and digestive issues in Europe since the 1950s. Calcium Carbonate is a naturally occurring mineral and is added for it anti-caking properties to improve the consistency of the powder.
- Constable, Anne, et al. “An integrated approach to the safety assessment of food additives in early life: Locust (carob) bean gum.” Toxicology Research and Application 1 (2017): 14-15. http://journals.sagepub.com/doi/10.1177/2397847317707370
- Joint, F. A. O., World Health Organization, and WHO Expert Committee on Food Additives. Evaluation of certain food additives: eighty-second report of the Joint FA. World Health Organization, (2016): 21-28 http://apps.who.int/iris/bitstream/handle/10665/250277/9789241210003-eng.pdf?sequence=1
- Kawamura, Yoko for the 69th JECFA, revised by Dr. J. Smith, Ph.D. and reviewed by Dr. Madduri V. Rao, Ph.D. for the 82nd JECFA “Chemical and Technical Assessment (CTA), 2016: Carob Bean Gum.” FAO, (2016): 1-10. http://www.fao.org/3/a-br563e.pdf
- Meunier, Leo, et al. “Locust bean gum safety in neonates and young infants: an integrated review of the toxicological database and clinical evidence.” Regulatory Toxicology and Pharmacology 70.1 (2014): 155-169. https://www.sciencedirect.com/science/article/pii/S0273230014001470
Safety of Ingredients: Purathick
Purathick is contraindicated for children less than 1 year of age. Purathick is made from three simple hypoallergenic ingredients, organic tapioca maltodextrin, organic tara gum and calcium carbonate, all with a long history of safe use. Tapioca maltodextrin is an easily digestible polysaccharide, used as a food additive, derived from the cassava root. Tapioca maltodextrin is often found in hypoallergenic infant formulas, and maltodextrin is the most common source for carbohydrates in enteral formulas. Maltodextrin is also a common ingredient in commercial thickeners, though usually derived from corn or wheat. Purathick only uses maltodextrin derived from tapioca. Tara gum is the second ingredient in Purathick. Also known as Peruvian carob, tara gum is a naturally occuring seed gum, consisting of galactomannan-type polysaccharides and is structurally similar to guar gum and locust bean gum. Calcium Carbonate is a naturally occurring mineral and is added for it anti-caking properties to improve the consistency of the powder.
- Food Standards Australia New Zealand (FSANZ)(2016). Final Assessment Report: Application A546: Tara Gum as a Food Additive, 33-44. http://www.foodstandards.gov.au/code/applications/pages/applicationa546tarag2904.aspx
Other Links & Resources
Tobritzhofer, Brianna, “Senior Wellness: Latest Food Products for Dysphagia Patients” Today’s Dietitian Vol. 21, No. 3, P. 16 (March 2019). https://www.todaysdietitian.com/newarchives/0319p16.shtml
Intermountain Healthcare, Primary Children’s Hospital. “Thickening Agents: What You Need to Know” Patient Handout. (2015). https://intermountainhealthcare.org/ext/Dcmnt?ncid=528112015
Thickening Practices and MBS use, ListServ Summary, June 2017. http://iprc.info/wp-content/uploads/2017/06/Directors-Forum-Question-Thickening-and-MBS.pdf